Philips has issued the following field safety notice regarding the HS1 adult electrode pads (M5071A) and HS1 paediatric electrode pads (M5072A);
M5071A (adult) and M5072A (infant/child) pads for use with HS1/OnSite/Home AEDs may experience gel separation and reduction of gel surface area.
Please find a link to an FAQ page that will answer any questions you may have: https://www.usa.philips.com/healthcare/resources/landing/pads-medical-device-correction.
What is the problem and under what circumstances can it occur?
HS1 adult and paediatric pads (M5071A and M5072A) have been observed to experience gel separation from the foam/tin backing when peeled from the yellow plastic liner. The gel may fold onto itself resulting in reduced surface area of gel on the pad, or it may separate almost completely leaving only a small amount of gel on the pad.
Any pad currently installed or stored with a HS1 defibrillator could experience this problem, and it is not possible to know prior to using the device if your pads are affected due to the protective foil seal.
Philips has received 115 complaints about this issue since 2010 (of which 84 complaints were received in 2021) for a total of 5 million shipments of M5071A and M5072A pads. Users should continue to use the HS1/OnSite/Home AED and pads as-is, and follow the voice prompts because the device will talk the user through the necessary actions.
The Philips HS1 defibrillator is intended for use by minimally trained or untrained individuals to treat a person in Sudden Cardiac Arrest (SCA).
What is the hazard associated with the issue?
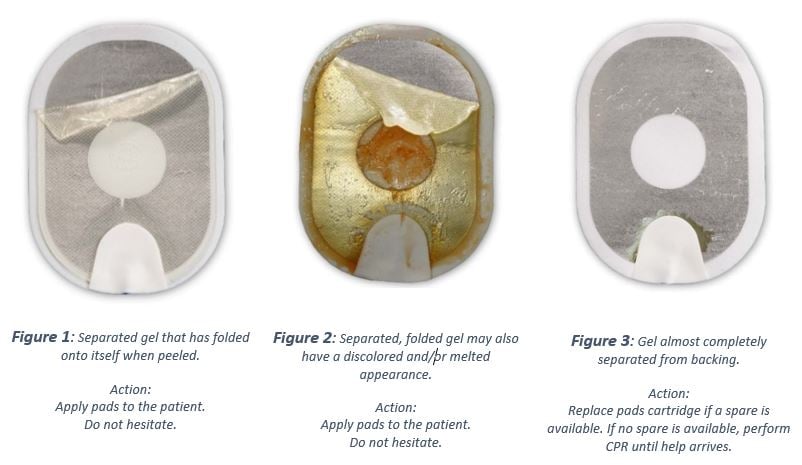
When a pad with separated, folded gel is placed on the patient's bare skin, the HS1 could deliver less effective or ineffective treatment due to the reduced surface contact area with the skin. See example picture in Figure 1.
Separated, folded gel may also have a discoloured and/or melted appearance. While the gel may also have a discoloured and/or melted appearance, the appearance does not have any impact on the delivery of therapy; however, there may be a delay in therapy if the user hesitates to apply the pad due to its appearance, and the device may deliver less effective or ineffective therapy due to the reduced contact area with the skin. See example picture in Figure 2.
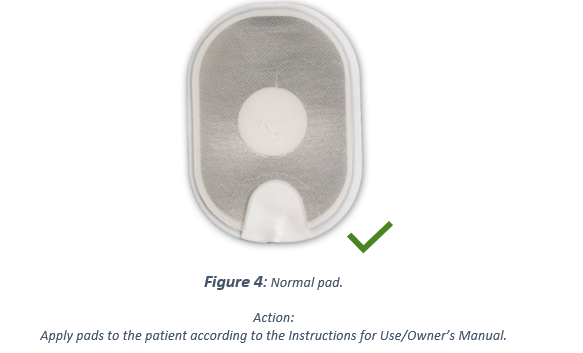
It is also possible that the gel could separate almost completely from the foam/tin backing when peeled, (see Figure 3.) Due to a small amount of gel surface contact area with the skin, electrical arcing could occur when a shock is delivered leading to burns to the patient's skin, or the AED could be unable to deliver any shock through the pads. A delay in therapy will result while the user installs a replacement pads cartridge (if available) or performs CPR while waiting for Emergency Medical Services Personnel to arrive. For comparison, Figure 4. shows a normal pad. No matter the state of the pad, follow the voice prompts because the defibrillator talks you through the necessary steps.
How do I identify affected products?
Affected products include all Lots of Adult and Infant/Child Pads Cartridges M5071A (adult) and M5072A (infant/child) installed in or stored as spares with the HS1. this notice takes into consideration only pads that are unexpired. Note, subsequent shipments will still be affected until updated pads are available.
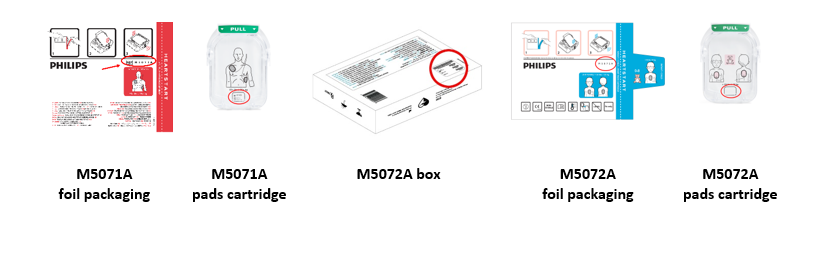
The M5071A and M5072A part numbers are located on the pads cartridge and the foil packaging. The M5072A identifier can also be found on the box that Infant/Child pads are shipped in. See photos below with the location of the part number circled.
What actions should customers take to prevent risks?
Continue using the HS1/OnSite/Home AED and pads as-is. During use, ensure the majority of the pad surface is covered with gel and apply the pads to the chest. If you notice the gel beginning to separate from the foam backing as you peek, try to prevent the gel from folding onto itself if possible. Do not hesitate to apply the pads to the chest unless the gel has almost completely separated from the backing as in Figure 3. No matter the state of the pads, follow the voice prompts because the device talks you through the necessary actions.
Do not try to examine the pads gel prior to use. It is not possible to know if your pads are affected by the problem prior to use because the pads are protected by a foil seal. The foil seal on the pads cartridge should be opened only for patient use in an emergency because the pads dry if out the seal is broken.
What actions are being taken by the Philips Emergency Care and Resuscitation business to correct the problem?
Philips is actively working on design changes intended to eliminate the issue in the M5071A and M5072A pads. Philips projects to release updated pads later in 2022, dependent on design activities, subcomponent availability, and regulatory approvals. Philips plans to notify eligible customers and supply updated pads.
More Information
On behalf of Philips, we regret any inconvenience caused by this problem. If you need any further information or support concerning this issue visit https://www.usa.philips.com/healthcare/resources/landing/pads-medical-device-correction.
This notice has been reported to the appropriate Regulatory Agencies. Be sure to report any occurrence of this issue to Philips, your Philips representative, or to your local Regulatory authority.
defibshop are committed to equipping everyone with the skills and knowledge to save a life. Speak to one of our Product Specialists on 0161 776 7422 or fill out our Contact Form.






